3D LifePrints, a UK-based medical 3D printing specialist, has received clearance from the美国食品和药物管理局(FDA),因为他们EmbedMed个性化手术平台。
嵌入式允许进行手术计划过程的数字化,并实现快速的设计过程。它还使用特定于患者的医疗设备的3D打印来协助制造。FDA(510K)间隙是监管过程中的进一步步骤,该过程允许利物浦的公司(用FDA的话说)“证明要销售的设备是安全有效的,这是实质上等效的,到合法市场的设备。”
FDA(510k)间隙遵循3D LifePrints’ISO 13485 medical certification in 2021.
“This clearance is the first step in executing 3D LifePrints’ strategy to bring Personalized Surgery to the US,” said Scott Parazynski, 3D LifePrints US Strategy Director and former NASA astronaut.
联合创始人兼首席执行官Henry Pinchbeck表示:“我们的目标是使美国各地的外科医生直接获得个性化的外科手术计划和特定于患者的设备。”“大规模嵌入式作品;无论您是希望建立一个护理点3D设施的医院,还是想要为复杂案例的个性化设备的外科医生,我们高素质的生物医学工程师都在这里提供帮助。”
Parazynski added, “Our facility at the Texas Medical Center, which enables our engagement with hospitals such as Houston Methodist and MD Anderson will act as the blueprint for a nationwide rollout.”
手术门户的功能 - 嵌入
嵌入式功能包括通过图像扫描数据处理患者输入数据,该数据是术前软件工具。3D Lifeprints软件旨在简化手术计划过程。嵌入式手机,计算机和平板电脑可访问。该平台提供了增强的可视化功能,使临床医生能够审查和评估许多患者病例。
EmbedMed’s in-built analytics enable institutions to learn about improvements in patient outcomes as well as health and operational economics. Medical devices and patient-specific model orders can be placed by clinicians using EmbedMed. It also helps clinicians track their patient’s health progress via EmbedMed.
3D LifePrints receives patients’ scans, then digitally plans, and visualizes the surgery through their Cloud Digital Platform (CDP). This is followed by producing and delivering sterilizable surgical guides and anatomical models to hospitals. Patient-specific surgical guides are delivered within 5 days to your doorstep. Cranio-maxillofacial guides and implants are delivered within 3 weeks.
规范医疗3D打印部门
位于Department of Health and Human Services, the Food and Drug Administration (FDA or USFDA) is a federal organization. The FDA is in charge of ensuring the safety of food, tobacco products, dietary supplements, prescription and over-the-counter medications, vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, animal foods & feed, and veterinary products in order to protect and advance public health.
这Federal Food, Drug, and Cosmetic Act (FD&C)is the FDA’s major area of enforcement. The organization also upholds related regulations and other laws, most notably Section 361 of the Public Health Service Act. Previously the FDA released adiscussion paper on medical 3D printing.

FDA clearance is a critical milestone necessary for enterprises to access the market.Desktop Health由美国3D打印机制造商台式金属于2021年初推出。该公司的Flexcera Base resin received an FDA (510k) clearanceseveral months later. Elsewhere, FDA clearance was required byABCorp,在波士顿的增材制造中心(AMC)。Abcorp是为在医疗部门工作的金融部门服务的合同制造商。这AMC已在FDA注册permitting it to serve major medical device production firms handlingI级,II和一些III类products. Neil Glazebrook, VP of 3D Solutions at ABCorp said, “Additive manufacturing, including 3D printing, offers our clients complete design freedom and is nothing less than transformative for the medical device industry.”
提名3D打印行业Awards 2022have now commenced. Who do you think should make it to the shortlists for this year’s show? Nominate now, the form closes at the end of the month.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the3D打印行业newsletter或跟随我们Twitter, or liking our page onFacebook.
当您在这里时,为什么不订阅我们Youtube渠道?包括讨论,汇报,视频短裤和网络研讨会重播。
Are you looking for a job in the additive manufacturing industry? Visit3D打印作业在行业中选择一系列角色。
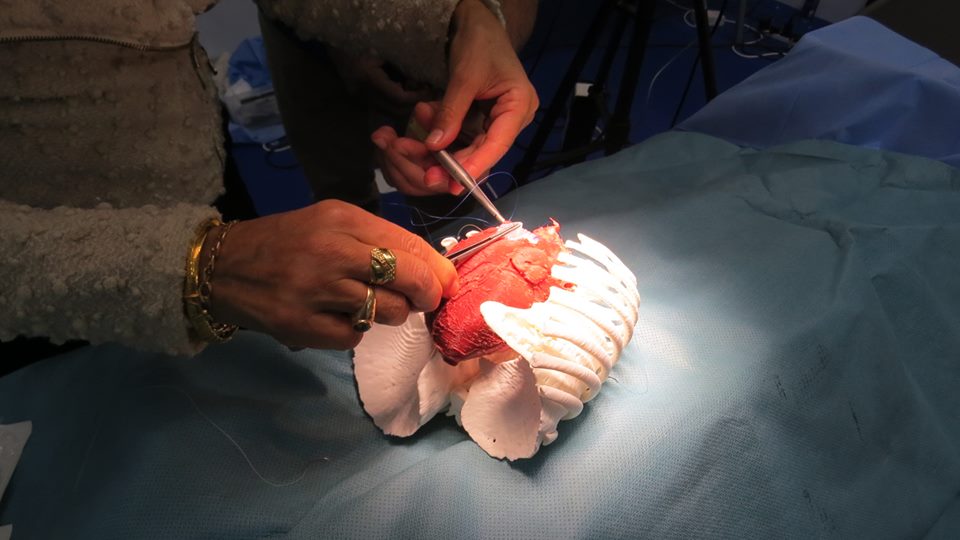
特色图像显示了3D打印心脏模型上的测试操作。通过3D LifePrints的照片


